
|

|
February 17, 1998
Next Electronics Breakthrough:
Power-Packed Carbon Atoms
By MALCOLM W. BROWNE
cientists experimenting with a fascinating speck of matter called a single-walled carbon nanotube predict that this elegantly geometrical molecule is about to ignite a revolution in electronics, computers, chemistry and new structural materials.
In place of the relatively large electronic devices incorporated in silicon-based chips, physicists have proved that it is possible to create devices on an atomic and molecular scale. A single electron in a single-wall carbon nanotube could function as a microminiature transistor.
Credit: Lawrence Berkeley National Laboratory Alex Zettl of Lawrence Berkeley laboratory holds a model of a carbon nanotube.
Nanotubes only a few atoms in diameter, which spontaneously form from hexagonal arrays of carbon atoms, were discovered in 1991 by Dr. Sumio Iijima of NEC Fundamental Research Laboratories in Tsukuba, Japan. These tubes, actually elongated molecules, form in furnaces from vapor generated by carbon arcs and lasers. They take their name from the nanometer, a unit of measurement one-billionth of a meter long -- a convenient length for specifying molecular dimensions.
Several recent reports show that nanotubes only one-50,000th the thickness of a human hair can perform the same electronic functions as vastly larger silicon-based devices. As a result, a computer based on nanotube devices could be extremely compact, fast and powerful.
Dr. Alex Zettl and his research group at the University of California at Berkeley recently showed that when two slightly dissimilar nanotube molecules are joined together end to end, the "junction" between them functions as an electronic device called a diode. Diodes are the basis of rectifiers, devices that are commonly used to convert alternating current into direct current.
"When we grow nanotubes," Dr. Zettl said, "electronic devices naturally form on them."
As ever smaller electronic devices are needed to improve the speed and power of computers, "the silicon industry is coming up against a brick wall," Dr. Zettl said. The solution may be to replace the silicon-based devices used today with minuscule carbon molecules, which would have another advantage: they conduct heat much faster than silicon, and therefore would be more suitable for microelectronics.
Looking farther into the future, Dr. Zettl suggested that clumps of carbon nanotubes might spontaneously organize their electronic interactions into complex webs analogous to the neural networks of the brain. The density of nanotube interconnections achieved by clumping them together is staggering; if all the nanotube carbon molecules that could be packed into a one-half-inch cube were laid end to end, they would extend 250,000 miles.
In a Few Atoms, Many Possibilities
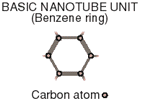
Nanotubes, molecules made mainly of carbon atoms, might one day replace silicon chips in computers much smaller than today's models. Nanotubes can be formed from condensed carbon vapor and they are 1/50,000th as thick as a human hair. Here are some types of nanotubes and their electrical properties.
CONDUCTOR
When the carbon rings line up with the main axis of a nanotube, the molecule conducts electricity as easily as if it were metal.
SEMICONDUCTOR

When the pattern of hexagonal rings in a nanotube is twisted, the nanotube acts like a semiconductor. That means it conducts electricity only after a certain threshold is reached.DIODE
When two nanotubes, one a conductor and one a semiconductor, are joined into one molecule, their junction acts like a diode, permitting electric current to flow only in one direction.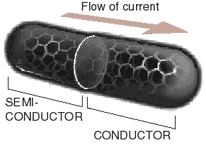
Source: University of California at Berkeley
Dr. Zettl speculated in an interview that a random jumble of nanotubes in such a cube could generate a network of nanocomputers that might be able to perform complex tasks and to reconfigure itself to improve its own efficiency.
Such a "tube cube," as Dr. Zettl calls the imaginary nanotube brain, may never materialize. But recent research offers strong evidence that nanotubes have, at least, a great electronic future.
Research reported last October by Dr. Zettl and his colleagues produced evidence that a single nanotube molecule could contain many tiny devices: transistors and other essential components of electronic systems.
Similar research is in progress at the National Aeronautics and Space Administration's Ames Research Center at Moffett Field in California. Dr. Jie Han and his group at Ames recently reported that by inserting defects into the junctions between metal-like nanotubes and semiconductor nanotubes, they had created a variety of junction types within a single nanotube molecule.
A pair of papers published last month in the journal Nature, one by chemists at Harvard University and the other by scientists at Delft University of Technology in the Netherlands and Rice University at Houston, independently reported the discovery that the electronic properties of a nanotube depend on the molecule's twist.
Chemists describe the raw material of nanotubes as sheets of graphite only one atom thick that are condensed from carbon vapor. Carbon atoms linked together in graphite sheets spontaneously form a pattern resembling chicken wire. When such a sheet rolls itself into a tube so that its edges join seamlessly together, a nanotube is formed. In some cases concentric tubes form, one inside another. Usually, hemispherical caps form at the ends of each tiny tube, thereby closing it.
In their studies, the Dutch and Harvard teams used scanning tunneling microscopes, probes so fine that their tips consist of single atoms. Such a microscope can scan the surface of an object and detect individual atoms.
The group led by Dr. Cees Dekker in the Netherlands and the Harvard team headed by Dr. Charles M. Lieber both reported that there was a strong relationship between a nanotube's electronic properties and its diameter and degree of twist.
If the graphite sheet forming a nanotube is rolled up perfectly evenly, so that all its hexagons line up along the molecule's axis, the molecule conducts electricity as readily as if it were a metal. But if the graphite rolls up at a twisted angle, the resulting nanotube behaves as a semiconductor. That is, it conducts electricity only when electrical or other energy applied to it exceeds a certain value.
The scientists also found that there were certain twist angles that permitted the nanotube to conduct electricity almost as freely as it would with no twist.
Dr. Mildred S. Dresselhaus of the Massachusetts Institute of Technology predicted in 1992 on the basis of quantum theory that just such behavior would be observed in single-wall carbon nanotubes. Last week she described the reports published by the Dutch and Harvard groups, which confirmed her prediction, as "landmark papers."
She acknowledged in an interview that it might take time before practical applications of the discovery were fully exploited. "But remember, it took a while for the laser to come fully into its own," Dr. Dresselhaus said.
Simple carbon nanotubes have already found industrial uses. For example, the Hyperion Catalysis International Company of Cambridge, Mass., adds small amounts of these molecules to plastic to make the plastic electrically conductive. Conductive plastics are used by the automotive industry to make parts that are coated with electrically charged droplets of paint. This electrostatic painting process saves most of the paint otherwise wasted by conventional spraying and applies a more even coat.
The scientific journey that led to carbon nanotubes began in 1985 with a momentous discovery by Dr. Richard E. Smalley and Dr. Robert F. Curl of Rice University, and Dr. Harold W. Kroto of the University of Sussex in England. The three scientists, who shared the 1996 Nobel Prize in Chemistry for their achievement, proved the existence of soccer-ball-shaped molecules created by linking together 60 or more carbon atoms.
The electronic links between carbon atoms in these ball-shaped molecules form a geodesic structure resembling the architectural designs of R. Buckminster Fuller, and were therefore dubbed "buckyballs." Compounds made by chemically uniting such balls with other atoms or molecules are called "fullerenes."
Many laboratories have experimented with different methods for condensing buckyball molecules from hot carbon vapor, and scientists have found that small variations in processing conditions produce hollow, roughly spherical molecules of varying shapes and sizes. But only in 1991 did Dr. Iijima discover accidentally that similar methods also yielded tubular molecules. Moreover, under certain conditions, these tubes sealed themselves by joining with the two halves of a split buckyball as end caps.
Many chemists, physicists and materials scientists began studying these molecular tubes, and it was soon found that they are about 100 times as strong as steel. They are far too small to use as individual fibers, but some laboratories have assembled bits of "rope" made up of carbon nanotubes.
"Nanotube fibers are uniquely tough," Dr. Lieber of Harvard said. "It's certain that they will be an ingredient in a new family of composite materials that will be much stronger than existing fiber-reinforced composites."
Dr. Lieber, Dr. Smalley and others have suggested that carbon nanotubes could function as molecular test tubes filled with reactant chemicals and sealed at both ends. Nanotubes might also be used to deliver medication to specific parts of the body. Some scientists predict that fuel cells converting hydrocarbon fuels directly into electrical energy will incorporate membranes made of nanotubes.
One of the biggest challenges in developing technologies based on carbon nanotubes, scientists say, will be in finding ways to sort them out. The furnaces in which nanotubes are made produce them in bulk and many types of nanotubes are always jumbled together: twisted and untwisted tubes, multiwalled and single-walled versions, some capped and others open, some wide and others narrow.
Dr. Smalley sees the solution in chemistry.
"We have a bag of tricks to work with," he said. "For instance, you might chemically attach some molecule to the ends of the nanotubes you're interested in. Then, as all the nanotubes moved past some substance, the ones with the added molecule would stick and stay behind, while the others moved on.
"In the coming decade," Dr. Smalley said, "we're going to see the flowering of a new branch of organic chemistry based on carbon nanotubes. There's no end to its possibilities."
Related Site
The following link will take you to a site that is not part of The New York Times on the Web, and The Times has no control over its content or availability. When you have finished visiting this site, you will be able to return to this page by clicking on your Web browser's "Back" button or icon until this page reappears.

|